Multimdal nonlinear microscopy for biological imaging
1. In vivo imaging study of spinal cord injury
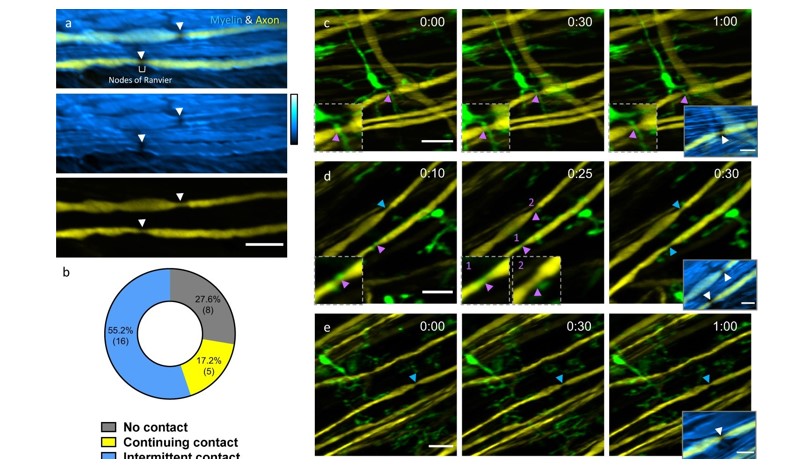
In vivo spinal cord imaging in mouse models without introducing immunological artifacts is critical to understand spinal cord pathology and discover effective treatments. We developed a minimally invasive intervertebral window by retaining the ligamentum flavum to protect the underlying spinal cord. By introducing an optical clearing method, we study neuron-glia dynamics following laser axotomy and observe strengthened contact of microglia with the nodes of Ranvier during axonal degeneration. By enabling long-term, repetitive, stable, high-resolution and inflammation-free imaging of mouse spinal cord, our method provides a reliable platform in the research aiming at interpretation of spinal cord physiology and pathology.
Related article:
Related research grants:
- General Research Fund (16102920): “Development of advanced multimodal nonlinear optical microscopy for in vivo study of spinal cord injury”
- Collaborative Research Fund (C6001-19EF): “High-resolution adaptive optics microscope system for live and deep imaging of biological tissues”
- Research Equipment Competition (REC14EG09): “High resolution stimulated Raman scattering (SRS) microscope system”
2. In vivo metabolic imaging and monitoring of adipose tissue
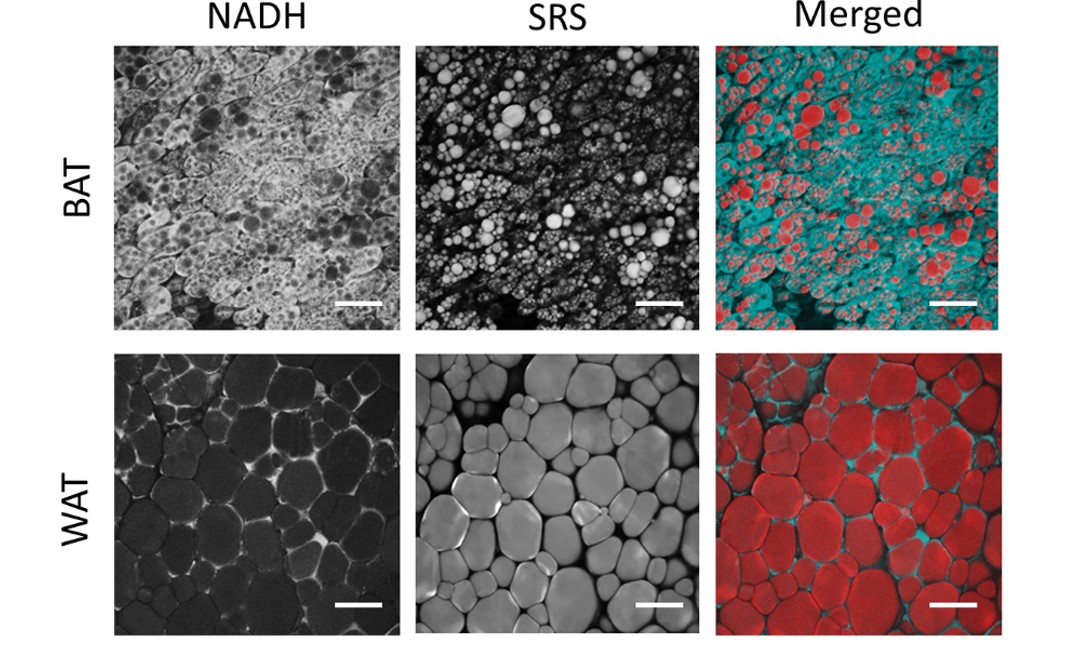
We investigated the metabolic characteristics of adipose tissues in live mouse model using a multiphoton redox ratio, fluorescence lifetime imaging technology and stimulated Raman scattering microscopy. Our study uncovered significant heterogeneity in the cellular structures and metabolic characteristics of thermogenic adipocytes in brown and beige fat. Subgroups of brown and beige adipocytes were identified based on the distinct lipid size distributions, redox ratios, fluorescence lifetimes and thermogenic capacities. The results of our study show that this label-free imaging technique can shed new light on in vivo study of metabolic dynamics and heterogeneity of adipose tissues in live organisms.
Related articles:
- Sicong He, Yitai An, Xuesong Li, Xiuqing Wei, Qiqi Sun, Zhenguo Wu, Jianan Y. Qu (2018).
“In vivo metabolic imaging and monitoring of brown and beige fat”. Journal of Biophotonics, 11(8), e201800019.(2018).
- Sicong He, Xiuqing Wei, Zhongya Qin, Congping Chen, Zhenguo Wu, Jianan Y Qu (2019).
“In vivo study of metabolic dynamics and heterogeneity in brown and beige fat by label-free multiphoton redox and fluorescence lifetime microscopy”. Journal of Biophotonics, 2019;e201960057.
Related research grants:
- General Research Fund (16102518): “Label-free imaging of adipose tissue in vivo with integrated stimulated Raman scattering and two-photon fluorescence microscopy”
- Research Equipment Competition (REC14EG09): “High resolution stimulated Raman scattering (SRS) microscope system”
3. Quantitative Imaging of Biological Dynamics by Stimulated Raman Scattering Microscopy
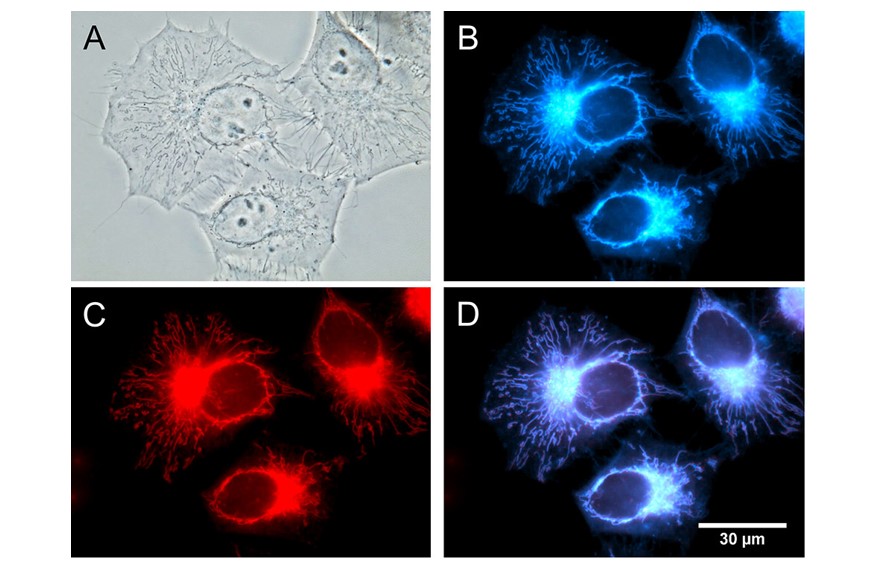
Quantitative methods to precisely measure cellular states in vivo have become increasingly important and desirable in modern biology. We developed a technique based on SRS microscopy of vibrational tags for quantitative imaging of lipid synthesis, lipolysis and biodistributions in live animals and cells. The technique aims to overcome the major limitations of conventional fluorescent and lipid extraction methods that do not provide the capability of in vivo quantitative analysis. Using a hyperspectral SRS (hsSRS) microscope and subtraction method, we demonstrated the unique capability of hsSRS microscopy in quantitative analysis of lipid metabolism in vivo. We reported a probe, named AIE-SRS-Mito, for imaging mitochondria in live cells via fluorescence (FL) and stimulated Raman scattering (SRS) imaging.
Related articles:
- Xuesong Li, Meijuan Jiang, Jacky W. Y. Lam, Ben Zhong Tang and Jianan Y. Qu,
“Mitochondrial Imaging with Combined Fluorescence and Stimulated Raman Scattering Microscopy Using a Probe of the Aggregation-Induced Emission Characteristic”, Journal of the American Chemical Society, Vol. 139, No.47, 17022-17030 (2017).
- Xuesong Li, Yan Li, Meijuan Jiang, Wanjie Wu, Sicong He, Congping Chen, Zhongya Qin, Yifei Qiu, Ben Zhong Tang, Ho Yi Mak, Jianan Y. Qu .
“Quantitative Imaging of Lipid Synthesis and Lipolysis Dynamics in Caenorhabditis elegans by Stimulated Raman Scattering Microscopy”. Analytical Chemistry, Vol.91 (3), 2279-2287 (2019).
Related research grants:
- General Research Fund (16102518): “Label-free imaging of adipose tissue in vivo with integrated stimulated Raman scattering and two-photon fluorescence microscopy”
- Research Equipment Competition (REC14EG09): “High resolution stimulated Raman scattering (SRS) microscope system”