High-Precision Tracing of Cell Dynamics and Their Interactions
1.High-precision single-cell tracing
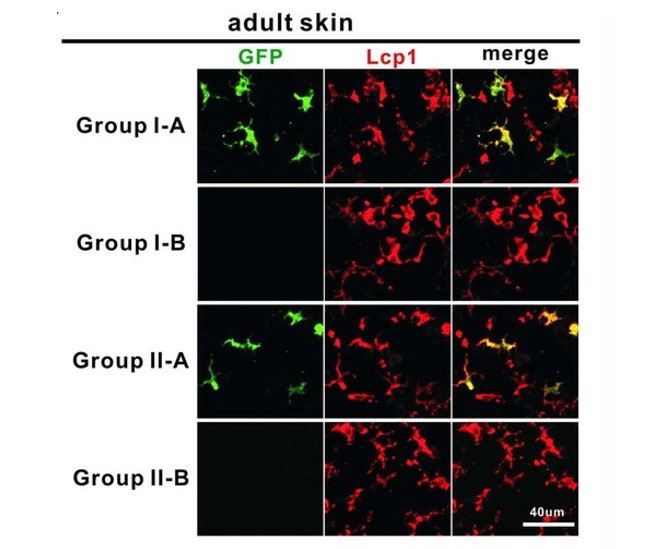
Heterogeneity broadly exists in various cell types both during development and at homeostasis. Investigating heterogeneity is crucial for comprehensively understanding the complexity of ontogeny, dynamics, and function of specific cell types. Traditional bulk-labeling techniques are incompetent to dissect heterogeneity within cell population, while the new single cell lineage tracing methodologies invented in the last decade can hardly achieve high-fidelity single-cell labeling and long-term in-vivo observation simultaneously. We developed a high-precision infrared laser-evoked gene operator heat-shock system to achieve precise single-cell labeling and tracing. In vivo study indicated that this system can precisely label single cell in brain, muscle and hematopoietic system in zebrafish embryo. Using this system, we traced the hematopoietic potential of hemogenic endothelium (HE) in the posterior blood island (PBI) of zebrafish embryo and found that HEs in the PBI are heterogeneous, which contains at least myeloid unipotent and myeloid-lymphoid bipotent subtypes.
Related articles:
- Sicong He, Ye Tian, Shachuan Feng, Yi Wu, Xinwei Shen, Kani Chen, Yingzhu He, Qiqi Sun, Xuesong Li, Jin Xu, Zilong Wen, Jianan Y Qu (2020).
“In vivo single-cell lineage tracing in zebrafish using high-resolution infrared laser-mediated gene induction microscopy”. eLife, 2020;9:e52024.
- Sicong He, Jiahao Chen, Yunyun Jiang, Yi Wu, Lu Zhu, Wan Jin, Changlong Zhao, Tao Yu, Tienan Wang, Shuting Wu, Xi Lin, Jianan Y Qu, Zilong Wen, Wenqing Zhang, Jin Xu .
“Adult zebrafish Langerhans cells arise from hematopoietic stem/progenitor cells”. eLife, Vol. 7 (2018).
- Jin Xu, Lu Zhu, Sicong He, Yi Wu, Wan Jin, Tao Yu, Jianan Y. Qu and Zilong Wen,
“Temporal-Spatial Resolution Fate Mapping Reveals Distinct Origins for Embryonic and Adult Microglia in Zebrafish”, Developmental Cell, V. 34, 632-641 (2015)
Related research grants:
- NSFC/RGC Joint Research Scheme (N_HKUST603/19): “Study of the lineage relationship between hematopoietic stem cells and tissue-resident macrophages using a high-precision single-cell tracing technique”
2. Satellite cell dynamics and interactions in muscle regeneration
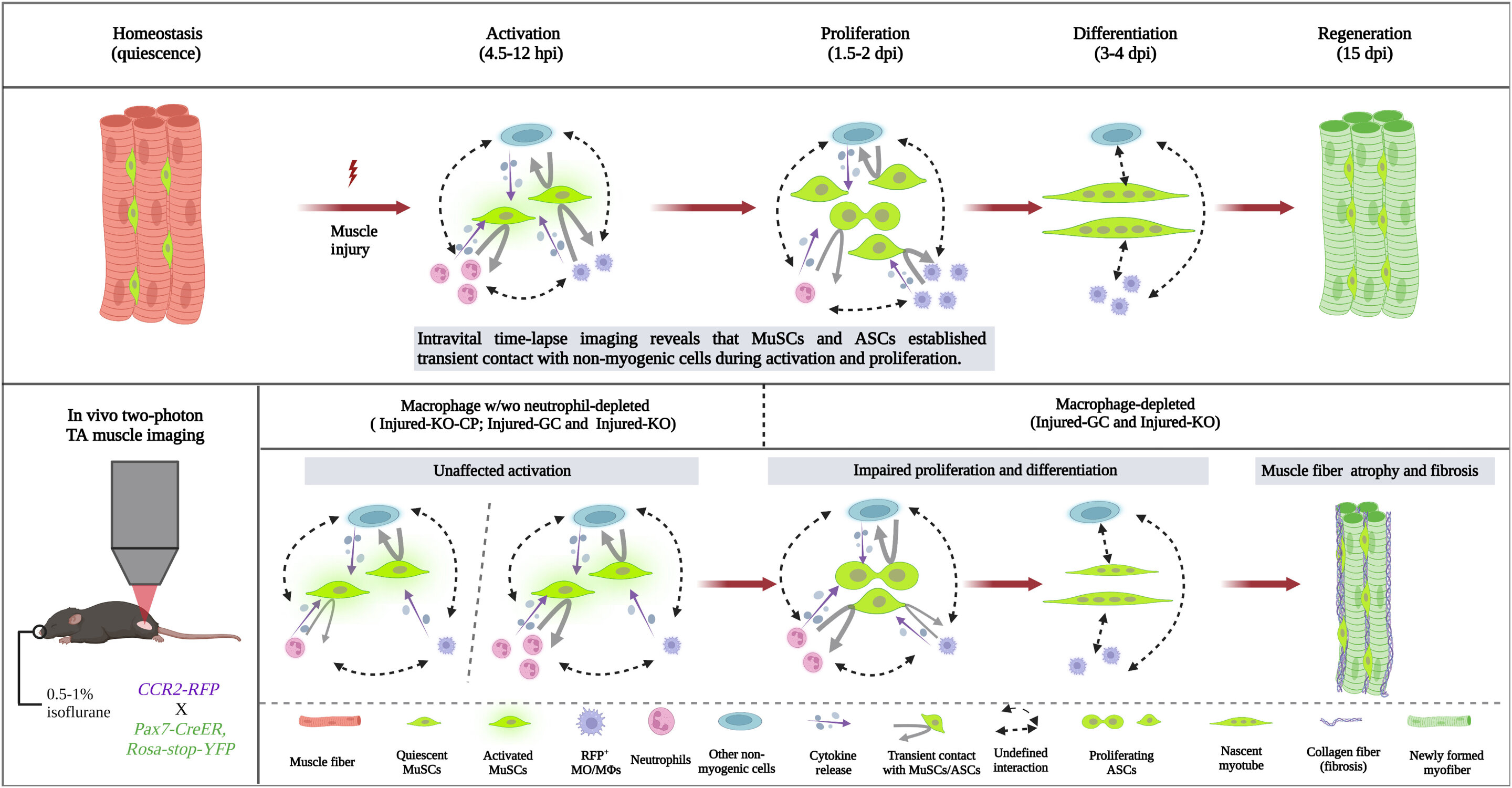
Skeletal muscle regeneration hinges on the coordinated efforts of muscle satellite cells (MuSCs) alongside various cellular components. Despite technical constraints, understanding the dynamic interactions between MuSCs and non-myogenic cells, notably myeloid cells, in live animals remains elusive. This study introduces a novel dual-laser multimodal nonlinear optical microscope platform to observe real-time interactions between MuSCs and non-myogenic cells during early muscle regeneration. Through 3D time-lapse imaging on live reporter mice, featuring red fluorescence protein (RFP)-labeled macrophages and yellow fluorescence protein (YFP)-labeled MuSCs, alongside autofluorescence tracking of reduced nicotinamide adenine dinucleotide (NADH), we monitored the spatiotemporal dynamics of their interactions. Our results suggest a transient nature of cell-cell contact between RFP+ macrophages/RFP- non-myogenic cells and YFP+ muscle stem/progenitor cells during activation and proliferation stages. Inhibiting macrophage infiltration revealed that direct contact between macrophages and MuSCs was dispensable for early MuSC activation but crucial for subsequent proliferation and differentiation, impacting intramuscular fibrosis. Neutrophil depletion in CCR2 deficient mice did not impede initial MuSC growth, shedding new light on myeloid cells' functions in muscle regeneration.
Related articles:
Related research grants:
- General Research Fund (16102518): “Label-free imaging of adipose tissue in vivo with integrated stimulated Raman scattering and two-photon fluorescence microscopy”
- Research Equipment Competition (REC14EG09): “High resolution stimulated Raman scattering (SRS) microscope system”
- Collaborative Research Fund (C6001-19EF): “High-resolution adaptive optics microscope system for live and deep imaging of biological tissues”